Our Culture & Our People
Heading here for accessibility
Solving Complex Healthcare Challenges in an Innovative & Inclusive Culture
Our Global Team of over 41,000 is united in purpose and unique in perspective, working together in an inclusive environment to help solve some of the world's complex healthcare challenges.
Just as we appreciate the variety of scientific approaches in clinical trials, we believe broad perspectives coming together in an inclusive workplace leads to innovative ideas, more fruitful collaboration and a vibrant culture.

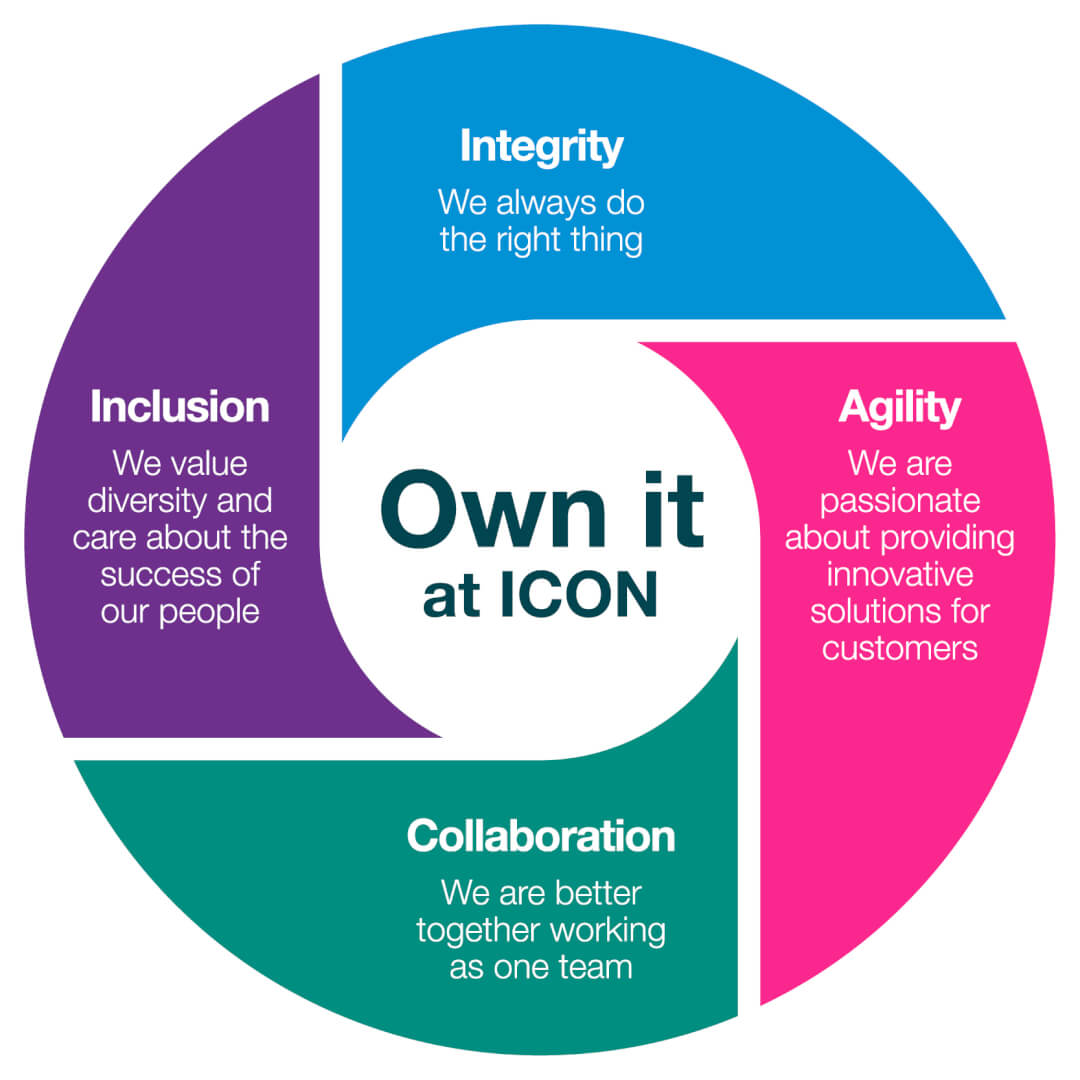
A vibrant culture grounded in four core values - Integrity, Collaboration, Agility, & Inclusion
As a values-driven organisation, integrity, collaboration, agility and inclusion are at the core of how we work and interact with each other, customers, patients and suppliers.
Our culture is a distillation of the best of what we have long been, combined with the best we can become. It gives us a shared sense of what we stand for individually and collectively.It's what will make us different as a company, enable us to become the partner of choice for our customers and help us to fulfil our mission to improve the lives of patients.
“Own it at ICON” is the philosophy that connects us to our values.
A culture of ownership means we own our customers’ challenges and we adopt a solutions-oriented approach to addressing them.
Our Own It culture encourages us to value diversity of thought and background in an inclusive workplace. It also inspires us to seize the career opportunities that are available as we become the world leader in healthcare intelligence.
Culture is not just what we do, it’s who we are.
“Own it at ICON” is the philosophy that connects us to our values.
A culture of ownership means we own our customers’ challenges and we adopt a solutions-oriented approach to addressing them.
Our Own It culture encourages us to value diversity of thought and background in an inclusive workplace. It also inspires us to seize the career opportunities that are available as we become the world leader in healthcare intelligence.
Culture is not just what we do, it’s who we are.


We own our responsibility
to make a difference to the communities
in which we live and work.

We own our responsibility to change the way clinical research works so that we can help make clinical research a care option for every patient.
We are committed to growing in a sustainable manner, conducting clinical research responsibly whilst minimising our environmental impact.
What Matters To You
Meaningful Work
Meaningful Work
ICON is an award-winning workplace that enables you to make a difference to patients’ lives by being part of a world-class clinical research organisation that helps deliver new medicines & medical devices that are benefitting patients worldwide.

Rewards &
Benefits
Rewards & Benefits
We offer competitive total rewards packages that are designed with your health, wealth and wellbeing in mind. Fair and equitable pay is core to our reward ethos, and we celebrate and reward high performance. Our benefits are focused on wellbeing and work-life balance for you and your family.
Career
Advancement
Career Advancement
Our success depends on the knowledge, capabilities and calibre of our people. That’s why we are committed to developing a continuous learning culture – one where we challenge you with engaging work and where every experience adds to your professional development.

Vibrant
Culture
Vibrant Culture
Our global team of over 40,000 is united in purpose and unique in perspective, working together in an inclusive environment to help solve some of the world’s most complex healthcare challenges. We value integrity, inclusion, collaboration and agility, which together form our ‘Own it’ culture that fosters innovative ideas and a vibrant workplace.
Our Awards
































Jobs for you at ICON
Salary
Location
Mexico, Mexico City
Location
Mexico City
Remote Working
Office Based
Business Area
ICON Full Service & Corporate Support
Job Categories
Clinical Data Management
Job Type
Permanent
Description
What you will be doing Assist Data Management Study Lead in maintenance of eCRF, Data Validation Specifications, and Study Specific Procedures.Manage clinical and third-party data reconciliation base
Reference
JR135148
Expiry date
01/01/0001

Author
Daniela Guerrero
Author
Daniela GuerreroSalary
Location
Brazil, Sao Paulo
Location
Sao Paulo
Remote Working
Remote
Business Area
ICON Strategic Solutions
Job Categories
Drug Safety
Job Type
Permanent
Description
We are currently seeking a Pharmacovigilance Associate to join our diverse and dynamic team. As a Pharmacovigilance Associate at ICON, you will play a vital role in monitoring and ensuring the safety
Reference
JR141138
Expiry date
01/01/0001

Author
Simone Chan
Author
Simone ChanSalary
Location
Brazil, Sao Paulo
Location
Sao Paulo
Remote Working
Remote
Business Area
ICON Strategic Solutions
Job Categories
Drug Safety
Job Type
Permanent
Description
We are currently seeking a Pharmacovigilance Associate to join our diverse and dynamic team. As a Pharmacovigilance Associate at ICON, you will play a vital role in monitoring and ensuring the safety
Reference
JR141137
Expiry date
01/01/0001

Author
Simone Chan
Author
Simone ChanSalary
Location
Brazil, Sao Paulo
Location
Sao Paulo
Remote Working
Remote
Business Area
ICON Strategic Solutions
Job Categories
Drug Safety
Job Type
Permanent
Description
We are currently seeking a Graduate Pharmacovigilance Associate to join our diverse and dynamic team. As a Graduate Pharmacovigilance Associate at ICON, you will play an essential role in supporting t
Reference
JR141135
Expiry date
01/01/0001

Author
Simone Chan
Author
Simone ChanSalary
Location
Poland, Warsaw
Location
Warsaw
Remote Working
Remote
Business Area
ICON Strategic Solutions
Job Categories
Clinical Trial Support
Job Type
Temporary Employee
Description
We are currently seeking a Clinical Trial Administrator to join our diverse and dynamic team. As a Clinical Trial Administrator at ICON, you will play a pivotal role in assisting with the design and a
Reference
JR141156
Expiry date
01/01/0001

Author
Zaklina Lomber
Author
Zaklina LomberSalary
Location
Brazil, Sao Paulo
Location
Sao Paulo
Remote Working
Remote
Business Area
ICON Strategic Solutions
Job Categories
Drug Safety
Job Type
Permanent
Description
We are currently seeking a Pharmacovigilance Associate to join our diverse and dynamic team. As a Pharmacovigilance Associate at ICON, you will play a vital role in monitoring and ensuring the safety
Reference
JR141140
Expiry date
01/01/0001

Author
Simone Chan
Author
Simone ChanImpactful work. Meaningful careers. Quality rewards.
At ICON, our employees are our greatest strength. That’s why we are committed to empowering you to live your best life, both inside and outside of work. Whether your ambition is lead a global team, become a deep scientific or technical expert, work in-house with our customers or gain experience in a variety of different ICON functions, we will support you in realising your full potential. Learn more about Our Culture at ICON
